
400 850 6500

2017-06-29 2273浏览
在学习A-level化学时,要学会总结题目中涉及到的化学反应类型,一点一滴地积累,从而做到积少成多。下面就是在A-level有机化学中涉及到的化学反应。
1. Alkane à chloroalkane
reagents: Cl2
conditions:
UV light
mechanism: free radical substitution
equation: RH +
Cl2 à RCl + HCl
2. Alkene
à polyalkene
Conditions: low T, high
p.
Equation:
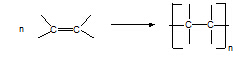
Type of reaction: addition
polymerisation (free radical)
3. Alkene à
bromoalkane
Reagent: HX(g)
Conditions: room
T
Equation:
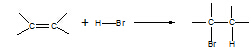
Type of reaction:
electrophilic addition
4. Alkene à
dibromoalkane
Reagent: Br2 in water or in an organic
solvent
Conditions: room T
Equation:
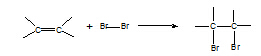
Mechanism: electrophilic
addition
5. Alkene à
alkylhydrogensulphate
Reagent: concentrated sulphuric
acid
Conditions: cold
Equation:
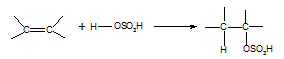
Mechanism:
electrophilic addition
6. Alkylhydrogensulphate
à alcohol
Reagent: water
Conditions:
warm
Equation:
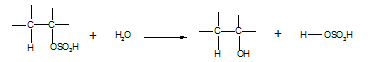
Type of reaction:
hydrolysis
7. Alkene à
alcohol
Reagent: steam
Conditions: 300 oC, 60 atm,
H3PO4 catalyst
Equation:
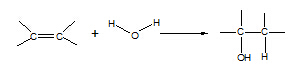
Type of reaction:
hydration
8. Haloalkane à
alcohol
Reagent: NaOH(aq) or KOH(aq)
Conditions: warm
under reflux
Equation: R-X + OH- à R-OH + X-
Type of reaction: nucleophilic substitution
9. Haloalkane
à nitrile
Reagent: KCN in aqueous
ethanol
Conditions: boil under reflux
Equation: R-X + CN- à
R-CN + X-
Type of reaction: nucleophilic
substitution
10. Haloalkane à Amine
Reagent: ammonia in
ethanol in a sealed tube
Conditions: heat
Equation: R-X + 2NH3 à R-NH2 + NH4X
Type of reaction: nucleophilic
substitution
11. Haloalkane à alkene
Reagent: KOH in ethanol
Conditions:
heat
Equation:
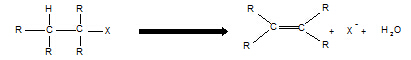
Type of reaction:
elimination
12. Primary alcohol
à aldehyde
Reagent: potassium
dichromate and dilute sulphuric acid
Conditions: warm,
distillation
Equation: RCH2OH + [O] à RCHO +
H2O
Type of reaction: mild oxidation
13.
Secondary alcohol à ketone
Reagent: potassium dichromate and dilute sulphuric
acid
Conditions: heat, distillation
Equation:
R1CH(OH)R2 + [O] à R1COR2 +
H2O
Type of reaction: oxidation
14. aldehyde
à carboxylic acid
Reagent:
potassium dichromate and dilute sulphuric acid
Conditions: heat,
reflux
Equation: R-CHO + [O] à R-COOH
Type of reaction:
oxidation
15. Alcohols à alkenes
Reagent: concentrated sulphuric acid
Conditions:
heat
Equation:
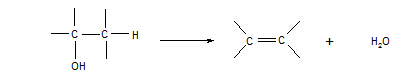
Type of reaction:
elimination
16. glucose à ethanol
reagent: yeast
conditions: 35 – 55
oC, no air
equation:
C6H12O6 à 2C2H5OH +
2CO2
type of reaction: fermentation
上一篇 : A-level物理——这些基本词汇你都认识吗?
下一篇 : A-level选择哪些科目有利于申请热门专业?
A/OLevel
![]() 苏公网安备32011302322644号增值电信业务经营许可证:苏B2-20190120 苏ICP备17009794号-16 Powered by marler.cn
苏公网安备32011302322644号增值电信业务经营许可证:苏B2-20190120 苏ICP备17009794号-16 Powered by marler.cn
2017-2021 AOLEVEL.COM.CN All Right Reserved.南京课窝教育科技有限公司版权所有